A 7.5 L vessel contains 10.0g of NO2 (g)and 0.55g of N2O4 (g) at equilibrium,as follows
2 NO2 (g) 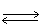 N2O4 (g)
N2O4 (g)
Calculate the value of the equilibrium constant for this reaction.
Definitions:
Stereotypes
Widely held but fixed and oversimplified image or idea of a particular type of person or thing.
Positive Assumptions
The concept of maintaining an optimistic outlook and expecting good outcomes, often leading to positive behavior and attitudes towards life and challenges.
Negative Assumptions
Cognitive biases or beliefs that lead an individual to view situations or outcomes more pessimistically, potentially contributing to anxiety and depression.
Cognitive Dissonance
The unease experienced mentally by an individual who possesses two or more contradictory beliefs, ideas, or values at the same time.
Q2: A solution of sodium acetate in water
Q6: Calculate the volume (in mL)of a 0.300
Q23: In the following equilibrium,as HNO<sub>3</sub> is added,the
Q47: Which phase change corresponds to evaporation?<br>A)Solid to
Q47: Which are the two Bronsted-Lowry acids in
Q48: Prepare a chart in which you name
Q56: Which has a definite volume but no
Q61: The mathematical expression for the equilibrium constant
Q93: Gamma radiation has a charge of<br>A)0<br>B)-1<br>C)+2<br>D)+4
Q98: In the following equilibrium;as the pressure is