In a closed reaction vessel having a volume of 1.00 L,1.25g of N2O4 are allowed to undergo the following reaction.
N2O4 (g) 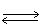 2 NO2 (g)
2 NO2 (g) 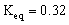 What will be the equilibrium concentrations of NO2(g)and N2O4(g)in moles/Liter?
What will be the equilibrium concentrations of NO2(g)and N2O4(g)in moles/Liter?
Definitions:
Primary Material
The main raw materials used in the production of goods or services, often a critical component in manufacturing processes.
Indirect Labor
Labor costs not directly tied to the production of goods or services, such as salary of supervisory staff.
Depreciation
The systematic allocation of the cost of a tangible asset over its useful life to reflect its decrease in value over time.
Store Equipment
Fixed assets specifically purchased for use in a retail store setting, such as shelving, cash registers, and display units.
Q14: Carbon tetrachloride is a hydrocarbon.
Q19: As the volatility of a liquid increases,its
Q24: Which cavity contains the heart?<br>A)cranial cavity<br>B)vertebral cavity<br>C)abdominal
Q32: A 3.00 L sample of a gas
Q40: What is the vapor pressure of water
Q49: Name the structural levels of the body
Q65: A 4.00 L sample of a gas
Q85: In a voltaic cell,reduction occurs at the<br>A)External
Q88: Which anion will not precipitate silver ion?<br>A)Chloride<br>B)Nitrate<br>C)Bromide<br>D)Carbonate
Q95: Which is a carboxylic acid?<br>A)CH<sub>3</sub>OH<br>B)HCOOH<br>C)CH<sub>3</sub>OCH<sub>3</sub><br>D)CH<sub>4</sub>