Balance the following redox reaction is acidic solution and calculate the mass of iodine produced from the reaction of 100.0 mL of an aqueous solution that is 0.125 M in 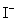 with 100.0 mL of a an aqueous solution that is 0.125 M in
with 100.0 mL of a an aqueous solution that is 0.125 M in 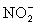 .
. 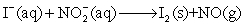
Definitions:
Capital Market Expectations
The forecasted future returns, volatilities, and correlations for the major asset classes, which are essential for the strategic asset allocation process.
Investment Objectives
Define the financial goals and strategies of an investor or an investment fund.
Risk
The potential for loss or unfavorable outcomes in any given situation, often assessed in finance as the variability of returns on investments.
Return
Return refers to the gain or loss on an investment over a specific period, including income received and the change in value, usually expressed as a percentage of the investment’s initial cost.
Q10: Which describes the study of the functions
Q12: Which is a pure substance existing as
Q27: Freezing point depression,boiling point elevation,and osmotic pressure
Q32: An 18.0 g sample of liquid water
Q37: " Life on Earth originated from the
Q43: Which of the following solutions will have
Q59: The term that best describes the rise
Q69: Describe the anatomical position.
Q77: An antigen is anything that can cause
Q93: What is the concentration of a H<sub>2</sub>SO<sub>4</sub>