Balance the following redox reaction is acidic solution and calculate the mass of iodine produced from the reaction of 100.0 mL of an aqueous solution that is 0.125 M in 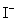 with 100.0 mL of a an aqueous solution that is 0.125 M in
with 100.0 mL of a an aqueous solution that is 0.125 M in 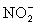 .
. 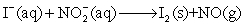
Definitions:
Chair Conformations
A term used in organic chemistry to describe the most stable form of a cyclohexane ring, resembling a chair, due to minimal steric strain.
Axial Methyl
A methyl group attached to a cyclohexane ring in a position parallel to the axis of the ring, leading to higher steric strain compared to equatorial positions.
Equatorial Methyl
A methyl group bonded to a carbon atom in a cyclohexane ring being in a position that extends outward from the perimeter of the ring, reducing steric strain.
Axial Positions
Locations on a molecule, especially in cyclic compounds like cyclohexane, that are perpendicular to the plane of the ring, typically leading to higher steric strain.
Q14: A solution has a lower vapor pressure
Q21: What is the boiling point of a
Q25: Which type of chemical bond involves the
Q39: What types of molecules are combined to
Q50: Which of the following diagrams represents an
Q54: What is the concentration of calcium ion
Q57: What mass of ice,at 0.00<sup> </sup><sup>°</sup><sup> </sup>C,can
Q67: Which subspecialty of physiology deals with the
Q75: Which are the four major elements found
Q76: Breeder reactors<br>A)manufacture fuel.<br>B)make use of nuclear fission.<br>C)use