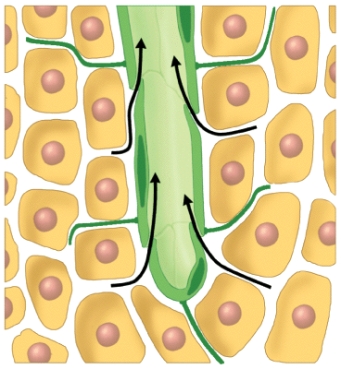
What process does this diagram represent?
Definitions:
Acid-Base Reaction
A chemical reaction that involves the transfer of a proton (H+) from an acid to a base, resulting in the formation of a conjugate base and a conjugate acid.
Equilibrium
The state in which the concentrations of reactants and products in a reversible chemical reaction are unchanged over time, indicating a balance in the rate of forward and reverse reactions.
Synthetic Route
The planned sequence of chemical reactions used to convert starting materials into desired products in the laboratory or industry.
Reagents
Chemical substances that are employed to bring about a chemical reaction in a provided set of reactants.
Q1: Capillaries are also referred to as<br>A)exchange vessels.<br>B)vasoconstrictors.<br>C)vasodilators.<br>D)pressure
Q2: A patient's blood pH is 7.48;partial pressure
Q12: Which class of antibodies is mainly found
Q25: The anterior pituitary develops from which of
Q29: What is a portal vein? Describe the
Q40: During embryonic development,blood cells are formed from<br>A)endodermal
Q53: Which of the following induces the production
Q65: A patient is on a diuretic (water
Q72: Why is the optic disc known as
Q74: Glycolysis,formation of acetyl CoA,Krebs cycle and the