During a Metabolic Pathway the Following Reaction Produced 3 G°' for the Hydrolysis of ATP Is -7
During a metabolic pathway the following reaction produced 3.00 moles of product.If G°' for the hydrolysis of ATP is -7.3 kcal/mol,how much energy would be involved? 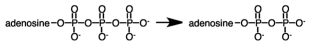
Definitions:
Incremental Value
The additional value created by undertaking a specific project or action, measured by the difference in a firm's value with and without the undertaking.
Outstanding Stock
The total shares of a corporation that are currently owned by all its shareholders, including shares held by institutional investors and restricted blocks held by insiders and company officers.
Vertical Acquisition
The purchase of a company that operates within the same industry but at a different level of the supply chain.
Q7: The region of an enzyme where the
Q13: Because many amino acids,fatty acids and carbohydrates
Q18: Bicarbonate ions which migrate from red blood
Q30: The principal anion in plasma is <img
Q43: The first step of the fatty acid
Q45: The medical condition that can result from
Q52: The function of this cellular organelle is
Q69: Which of the following has the highest
Q81: The type of enzyme that would catalyze
Q82: DNA and RNA are polymers produced from