During a Metabolic Pathway the Following Reaction Produced 3 G°' for the Hydrolysis of ATP Is -7
During a metabolic pathway the following reaction produced 3.00 moles of product.If G°' for the hydrolysis of ATP is -7.3 kcal/mol,how much energy would be involved? 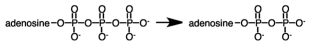
Definitions:
Dopaminergic Effect
An effect or action related to dopamine, a neurotransmitter involved in reward, motivation, memory, attention, and even regulating body movements.
Muscle Movement
The action or process of moving parts of the body by contraction and relaxation of muscles, crucial for all forms of physical activity and coordination.
Arise Slowly
The recommendation or act of standing up gradually to prevent dizziness or falls, often applicable to individuals with blood pressure issues.
GABA
Gamma-aminobutyric acid (GABA) is the primary inhibitory neurotransmitter in the brain, playing a crucial role in regulating neuronal excitability and involved in anxiety reduction and sleep regulation.
Q3: The modified internal rate of return corrects
Q3: The function of this cellular organelle is
Q11: It is considered important to suppress ones
Q13: One function of carbohydrates is to form
Q37: The following reaction would occur during fat
Q37: When the compound below is treated with
Q43: A firm has experienced a constant annual
Q68: Glucogenic amino acids can be converted into
Q69: Which of the following can be used
Q76: Perhaps the greatest disadvantage of using the