Multiple Choice
The relation PV = nRT holds for all ideal gases.The additional relation PVγ holds for an adiabatic process.The figure below shows two curves: one is an adiabat and one is an isotherm.Each starts at the same pressure and volume.Which statement is correct? (Note: "∝" means "is proportional to". ) 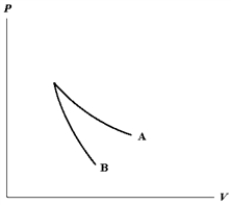
Definitions:
Related Questions
Q4: What is the potential difference V<sub>B</sub> −
Q6: The electric potential inside a charged solid
Q8: A boat is floating in a small
Q21: Charge of a uniform density (8.0 nC/m<sup>2</sup>)is
Q33: A constant volume gas thermometer has a
Q33: The figure below shows a planet traveling
Q38: If y = 0.02 sin (30x −
Q44: A nonconducting sphere of radius 10 cm
Q66: The electric potential at the surface of
Q69: Points A [at (2,3)m] and B [at