The relation PV = nRT holds for all ideal gases.The additional relation PVγ holds for an adiabatic process.The figure below shows two curves: one is an adiabat and one is an isotherm.Each starts at the same pressure and volume.Which statement is correct? (Note: "∝" means "is proportional to". ) 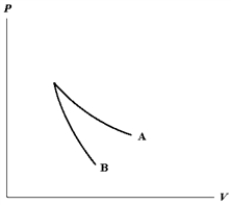
Definitions:
Extrinsic Motivations
Inspiration fueled by outside incentives, including financial gain, recognition, academic achievement, and commendation.
Overjustification Effect
A phenomenon where providing an external incentive for a behavior that is already internally rewarding can lead to a decrease in intrinsic motivation for that behavior.
Intrinsic Motivation
The drive to engage in an activity for its own sake, deriving pleasure and satisfaction from the activity itself rather than external rewards.
Secondary Motivation
The drive to perform an action or engage in behavior based on external rewards or pressures, rather than intrinsic or personal desires.
Q9: Star A has a radius of 200
Q10: The electric field in the region of
Q17: A merry-go-round of radius R = 2.0
Q42: A student has written the equation below
Q43: Determine the energy stored in C<sub>1</sub> when
Q43: Two harmonic waves are described by <img
Q44: A satellite of mass m circles a
Q52: The Bohr model pictures a hydrogen atom
Q62: A charge of 4.0 nC is distributed
Q91: Charge of uniform density 90 nC/m<sup>3</sup> is