How fast must two deuterium atoms be moving so they can overcome the Coulomb force of repulsion,and attain the necessary 10−14 m for fusion? (m( 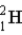
) = 2.014 1 u)
Definitions:
Electron Pairs
Refers to two electrons occupying the same orbital in an atom or molecule, usually forming a covalent bond or participating in lone pairs.
Confidence Interval
A selection of values, based on sample statistics, forecasted to incorporate the value of an unknown population parameter.
Population Mean
Population mean is the average of all the values in a population.
Standard Deviation
A statistic that measures the dispersion or variation of a dataset relative to its mean, indicating how spread out the data points are.
Q2: Nico Trading Corporation is considering issuing long-term
Q8: What,approximately,are the dimensions of the smallest object
Q16: Since the net proceeds from sale of
Q33: A uniform film of oil (n =
Q36: A single slit of width a is
Q37: The difference between the return on the
Q126: The _ of an asset is the
Q161: Nico bought 100 shares of Cisco Systems
Q177: Another term sometimes applied to a common
Q186: On average, during the past 75 years,