Consider the various frequencies of the three photons emitted from the following three individual electron transitions in the figure below: n=3 to n=2; n=2 to n=1; n=3 to n=1. These transitions would produce three spectral lines in a spectroscope. If the energy spacing between the levels were equal, would this affect the number of spectral lines? 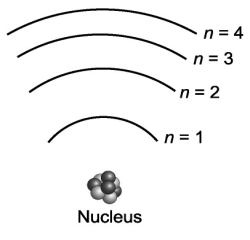
Definitions:
Q12: Magnetic domains normally occur in<br>A)iron.<br>B)copper.<br>C)silver.<br>D)all of the
Q18: Oxygen, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4071/.jpg" alt="Oxygen, ,
Q44: Which of the following intermolecular forces best
Q57: What is a hydrogen bond?<br>A)a special type
Q60: Which type of radiation-alpha, beta, or gamma-results
Q91: An iron rod becomes magnetic when<br>A)positive ions
Q95: If three samples have the half-lives listed
Q96: Magnet A has twice the magnetic field
Q107: Distant dark-colored hills appear blue due to
Q108: How does the wave model of electrons