Consider the boiling points of the following compounds and their solubilities in room-temperature water. Why does the solubilities in water go down as the boiling points of these alcohols go up. 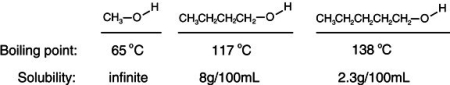
Definitions:
Only Child
A child who has no siblings, neither brothers nor sisters.
First Marriage
The initial legal, religious, or social union between two individuals as spouses.
Young People
Individuals in their early life stages, generally considered to be in the age range from adolescence to early adulthood.
Careers
The progression of occupational roles or jobs that a person undertakes over their working lifetime, often related to their skills and interests.
Q1: Why might a high-formula-mass alcohol be insoluble
Q2: Which of the following elements is a
Q16: Which equations are balanced? <br>A.Mg (s)+ 2HCl
Q16: Which are closer together: the two nuclei
Q19: Which of the following statements is untrue
Q22: If a material has a half-life of
Q26: Which of the following statements best describes
Q49: How is it possible for a neutral
Q83: What is the number of moles of
Q113: Which of the following elements is a