The boiling point of 1,4-butanediol is 230°C. Would you expect this compound to be soluble or insoluble in room-temperature water? 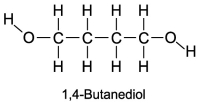
Definitions:
Forward Contracts
Customized contracts between two parties to buy or sell an asset at a specified price on a future date, used for hedging or speculation.
Futures Contracts
Agreements for the future delivery of assets like commodities or securities at a price fixed upon the contract's signing.
Option Contracts
Financial derivatives that give the buyer the right, but not the obligation, to buy or sell an underlying asset at a specified price on or before a certain date.
Swap Contracts
Financial agreements between two parties to exchange cash flows or other financial instruments for a specified period of time.
Q9: Which of the following molecules contains a
Q14: Why might the following nuclear reaction not
Q23: If it takes three carbon atoms to
Q24: Why is it important for a chemist
Q52: The following statement describes which subatomic particle
Q61: Why can't the elements of a compound
Q96: Which of the following has the greatest
Q105: Which of the following would be considered
Q138: What do the brackets in the following
Q142: Why do we use the pH scale