Consider the boiling points of the following compounds and their solubilities in room-temperature water. Why does the solubilities in water go down as the boiling points of these alcohols go up. 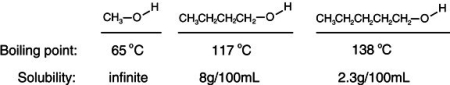
Definitions:
Decisions
The process of making choices or conclusions after considering various alternatives and their potential outcomes.
Aphasia
A condition characterized by the loss of ability to understand or express speech, caused by brain damage.
Cerebral Cortex
The outer layer of the cerebrum, playing a key role in memory, attention, perception, cognition, awareness, thought, language, and consciousness.
Broca's Area
A region in the frontal lobe of the dominant hemisphere (usually the left) of the brain with functions linked to speech production.
Q3: What is a chemical reaction?<br>A)when one or
Q29: Which statement best describes a compound?<br>A)a material
Q31: Which of the following would cost the
Q52: Why might disposing of a lead-acid battery,
Q72: Given the following energy profiles, which of
Q79: Which of the following statements about electrons
Q85: Which of the following provides the minimum
Q102: Which of the following diagrams best represents
Q106: Which would you expect to have a
Q138: What do the brackets in the following