The boiling point of 1,4-butanediol is 230°C. Would you expect this compound to be soluble or insoluble in room-temperature water? 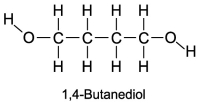
Definitions:
Feudalism
A social system in medieval Europe where the nobility held lands from the Crown in exchange for military service, and vassals were in turn tenants of the nobles, while the peasants were obligated to live on their lord's land and give him homage, labor, and a share of the produce.
American Opinion
Refers to the collective attitudes or beliefs held by the American public on various topics and issues.
Poverty
A socio-economic condition characterized by a lack of financial resources and access to basic needs such as food, shelter, and healthcare.
Socioeconomic Status
A combined measure that typically includes a person's income, education level, and occupation to classify their position within society.
Q27: When <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4071/.jpg" alt="When Po
Q38: Which of the following professions would likely
Q39: What is indicated by a gasoline's octane
Q51: Hydrogen sulfide, H<sub>2</sub>S, burns in the presence
Q72: The main component of bleach is sodium
Q95: Consider the appearance of an American flag
Q98: Given the following generic chemical reaction, which
Q103: What are the three primary colors for
Q112: For the above energy profiles, which reaction
Q128: An object having no real color may