One mole of a monatomic ideal gas at a pressure of 3.0 atm and a volume of 30 L is isothermally expanded to a pressure of 1.0 atm and a volume of 90 L.Afterwards it is compressed at a constant pressure until its volume is 30 L and then it's pressure is increased at a constant volume back to the original volume of 30 L as shown in the PV diagram.What is the efficiency of this heat engine cycle? 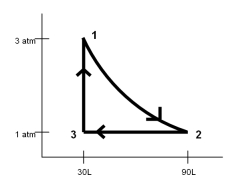
Definitions:
Windows
A graphical operating system developed by Microsoft, providing an environment for executing and managing applications.
Competing Firms
Competing firms are companies within the same industry or market that offer similar products or services, striving for customer patronage and market share.
Windows Monopoly
Refers to the dominant position of Microsoft Windows as the operating system on personal computers, often discussed in the context of antitrust law and competition.
Anticompetitive Means
Practices that undermine fair competition in the market, often leading to monopolies or oligopolies.
Q21: While enjoyable,rock concerts can be damaging to
Q35: A bartender mixes 0.1 liter of alcohol
Q38: Two moles of helium are initially
Q45: You have an RC circuit with the
Q52: Two infinite,uniformly charged,flat surfaces are mutually perpendicular.One
Q54: A 2.45-m long wire of uniform linear
Q61: A solid 20.0-g mass of aluminum is
Q63: A gas is in a cylinder
Q67: The amount of work done to move
Q69: What is the resistance of a