An ideal gas 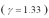 with a pressure of 1.00 atm at room temperature (300 K) is compressed from 500.0 cm3 to a final volume.The compression is adiabatic and the final pressure of the gas is 25.1 atm.What final volume of the gas?
with a pressure of 1.00 atm at room temperature (300 K) is compressed from 500.0 cm3 to a final volume.The compression is adiabatic and the final pressure of the gas is 25.1 atm.What final volume of the gas?
Definitions:
Perfectly Elastic
Describes a situation in economics where the quantity demanded or supplied changes infinitely in response to any change in price.
Interest-Rate Cost-Of-Funds
The cost incurred by financial institutions to raise funds that they can then lend to customers, influenced by prevailing interest rates.
Market Interest Rates
The prevailing rate at which borrowers and lenders agree to engage in transactions of debt securities in the financial markets.
Creative Destruction
A concept in economics which suggests that new innovations destroy old industries and methods, leading to new sectors and opportunities for growth.
Q14: A gas expands at constant pressure
Q15: A supertanker filled with oil has
Q44: Two pistons exert forces F<sub>1</sub> and F<sub>2</sub>
Q46: Which one of these circuits has the
Q49: In Rutherford's famous experiment,alpha particles of mass
Q56: In circuit shown,three identical light bulbs are
Q58: What is the period of a 1.30-m
Q59: In the figure,water flows in the pipe
Q61: I pull into a gas station
Q82: The diameter of an oxygen molecule is