For a class demonstration,your physics instructor pours 1.00 kg of steam at 100 C over 5.00 kg of ice at 0.00 C,allows the system to reach equilibrium and then he is going to measure the temperature of the system.While the system used in the class demonstration reaches equilibrium,you are given the latent heats of ice and steam and the specific heat of water to be,respectively: 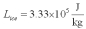 ;
; 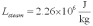 ;
; 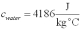 and you are asked to calculate the final equilibrium temperature of the system.What value did you find?
and you are asked to calculate the final equilibrium temperature of the system.What value did you find?
Definitions:
Scienter
A legal term referring to the knowledge of the wrongful nature of one's own act or omission.
Burden of Proof
The obligation to prove one's assertion or claim in a court of law.
Civil Case
A legal dispute between two or more parties in which compensation or specific performance is sought, rather than criminal sanctions.
Criminal Case
A legal proceeding brought against an individual or entity accused of breaking the law, where guilt must be proven beyond a reasonable doubt.
Q15: A point charge of 5 µC is
Q24: The potential energy stored in a
Q25: If you quadruple the radius of the
Q35: In order for a satellite to be
Q35: How long does it take for light
Q40: The potential energy of a system
Q53: A cylinder with a piston contains 2.00
Q57: A point charge + Q is located
Q59: A SUV has a length of 4.4
Q63: For an ideal gas,the temperature measured in