For a class demonstration,your physics instructor pours 1.00 kg of steam at 100 C over 5.00 kg of ice at 0.00 C,allows the system to reach equilibrium and then he is going to measure the temperature of the system.While the system used in the class demonstration reaches equilibrium,you are given the latent heats of ice and steam and the specific heat of water to be,respectively: 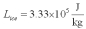 ;
; 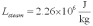 ;
; 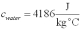 and you are asked to calculate the final equilibrium temperature of the system.What value did you find?
and you are asked to calculate the final equilibrium temperature of the system.What value did you find?
Definitions:
Consolidated Income Statement
A Consolidated Income Statement is a financial report that combines the income, expenses, and profits of a parent company and its subsidiaries, showing the overall performance as a single entity.
Acquisition Method
The Acquisition Method is an accounting technique used in consolidating the financial statements of a group where one entity controls others.
Noncontrolling Interest
The share of ownership in a subsidiary that cannot be directly or indirectly linked to the parent company.
Fair Value
The revenue expected from an asset sale or the cost to offload a liability in a transaction with market players on the date it is appraised.
Q6: What is the radius of a steel
Q10: In order to make a tight
Q14: A 2-m string with a mass of
Q14: A standing wave in a column of
Q35: A 7.2-kg mass oscillates on a horizontal
Q45: Consider the Earth to have a
Q55: A cylinder containing 5.00 mol of nitrogen
Q57: To crack a nut you exert equal
Q57: A point charge of 5.0 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1516/.jpg"
Q61: A traveling wave is represented by the