Which would you expect to have a higher melting point: sodium chloride, NaCl, or aluminum oxide, 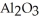 ?
?
Definitions:
Tractor-Trailer
A transportation vehicle consisting of a semi-tractor (the front part) and one or more trailers to carry freight.
Fourth Branch of Government
A term sometimes used to describe bodies or groups that have significant influence over public policy outside the traditional three branches of government.
DMV
Stands for Department of Motor Vehicles, a state-level government agency managing vehicle registration and driver licensing.
Tractor-Trailer
A large vehicle consisting of a towing engine or tractor attached to a trailer, commonly used for transporting goods over long distances.
Q17: How many bonds between nitrogen and hydrogen
Q17: Which molecule is most polar?<br>A) S=C=S<br>B) O=C=O<br>C)
Q19: Which of the following is a mixture?<br>A)
Q23: How are pressure and the boiling point
Q57: Uranium-235 releases an average of 2.5 neutrons
Q60: Oxygen, O, (number 8), sulfur, S, (number
Q111: If carbonic acid (H<sub>2</sub>CO<sub>3</sub>) were to undergo
Q130: Two amu equals how many grams? (1
Q138: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg" alt=" crystals are composed
Q145: Which element has the atomic number 12?<br>A)