Water, 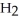 O, and methane, C
O, and methane, C 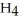 , have about the same mass and differ by only one type of atom. Why is the boiling point of water so much higher than that of methane?
, have about the same mass and differ by only one type of atom. Why is the boiling point of water so much higher than that of methane?
Definitions:
Initial Experimentation
The preliminary phase of testing or trying out a new method, idea, or activity to gather initial data or responses.
Final Application
The ultimate use or implementation of a product, concept, or technology in its intended environment or market.
Spontaneous Creativity
Refers to the ability to come up with new and original ideas without premeditation or external stimuli.
Feasibility Determination
The assessment process to evaluate whether a project or plan is possible and practical within its constraints.
Q5: Which of the following is a pure
Q38: Which of the following provides the minimum
Q65: Why do ice skates work?<br>A) The pressure
Q72: How many different elements are in the
Q74: A water bug can rest on top
Q76: Which of the following gases would diffuse
Q105: Does the hydride ion, H⁻, tend to
Q120: How many substituents surround the sulfur atom
Q123: The nucleus of an electrically neutral iron
Q124: Phosphate ions, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg" alt="Phosphate ions,