Which would you expect to have a higher melting point: sodium chloride, NaCl, or aluminum oxide, 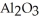 ?
?
Definitions:
Philippe Pinel
A French physician renowned for his contributions to the humane treatment of the mentally ill in the late 18th and early 19th centuries.
Mental Hygiene
The practice of maintaining mental health through the prevention and treatment of mental disorders, as well as by promoting healthy lifestyles and environments.
Antisocial Personality
A disorder characterized by a pervasive pattern of disregard for, and violation of, the rights of others.
Drug Abuse Disorder
A medical condition characterized by the compulsive use of drugs despite harmful consequences, leading to mental and physical health issues.
Q10: Where did the atoms that make up
Q17: The original reactor built in 1942 was
Q37: In a chemical equation the coefficients _.<br>A)
Q48: Which of the following statements is false?<br>A)
Q55: How many atoms of Oxygen (O) are
Q69: What is a probability cloud?<br>A) It is
Q69: The atomic masses appearing in the periodic
Q89: What would be the effect on an
Q118: Which of the following sources of radiation
Q127: If the solubility of a compound is