Consider the boiling points of the following compounds and their solubilities in room-temperature water. Why does the solubilities in water go down as the boiling points of these alcohols go up. 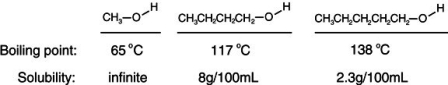
Definitions:
Superordinate Goals
Shared goals that override differences among people and require their cooperation to achieve.
Competing Objectives
Instances where objectives or goals of individuals, teams, or departments within an organization clash or oppose each other.
Unethical Negotiating Behaviour
Practices in negotiation that are dishonest, unfair, or violate moral principles.
Long-Run Losses
Financial deficits that a business incurs over an extended period, often leading to strategic reassessment or restructuring.
Q10: How does a base like NH<sub>3</sub> raise
Q54: When blue food coloring is dissolved in
Q67: List the following elements in order of
Q70: If an atom has 43 electrons, 56
Q73: Which of the following would cost the
Q78: If it takes 20 beryllium atoms to
Q120: Qualitatively, what happens to the hydroxide ion
Q129: The concept of a chemical bond is
Q138: When you increase the temperature of a
Q140: Rank the following by relative frequency from