Based on atomic size, which would you expect to be more soluble in water: helium, He, or nitrogen, 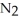 ?
?
Definitions:
Market Entry
The strategies or methodologies used by a company to start selling its products or services in a specific market or geographic area.
Direct Investment
Investment in a business or real estate venture in a foreign country in a way that grants the investor control or significant influence over the venture.
Internationalization
The process of designing products, services, and strategies in a way that they can be easily adapted to various international markets.
Global Marketplace
An interconnected environment where goods, services, and labor are exchanged across national borders, fueled by globalization.
Q30: What is the difference between a dipole-dipole
Q36: If the heat of melting of a
Q39: Why aren't solar stills used in more
Q43: Qualitatively, what happens to the hydronium ion
Q69: When the hydronium ion concentration equals 1
Q92: Mercury forms a convex meniscus with glass
Q95: How many grams of gallium are there
Q105: What is the molarity when water is
Q112: Which of the following statements about radiation
Q129: The concept of a chemical bond is