Consider the boiling points of the following compounds and their solubilities in room-temperature water. Why does the solubilities in water go down as the boiling points of these alcohols go up. 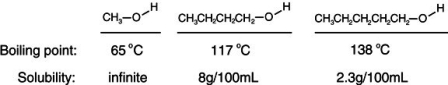
Definitions:
Product Complementarity
The degree to which two or more products enhance each other's use or value when used together.
Decorating Style
refers to the particular aesthetics and designs used to adorn and style interior spaces, reflecting personal tastes or cultural trends.
Lifestyles
The habits, attitudes, tastes, moral standards, economic level, etc., that together constitute the mode of living of an individual or group.
Brand Equity
The value that a brand adds to a product, often reflected in how consumers think, feel, and act with respect to the brand.
Q10: A sealed plastic bottle is filled with
Q25: Assume air has an average molar mass
Q30: For the following acid-base reaction, identify what
Q43: Which of the following is not a
Q57: What atom in the ammonium ion, <img
Q83: Which process would release energy from gold,
Q84: Since some of the compounds that are
Q87: Water, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg" alt="Water, O,
Q122: Why does liquid water expand slightly when
Q133: Which of the above illustrations shows an