Multiple Choice
Two chemical structures are shown, one of a typical gasoline molecule and the other of a typical motor oil molecule. Which is which? 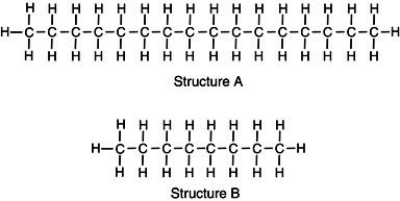 Base your reasoning not on memorization but rather upon what you know about molecular interactions and the various physical properties of gasoline and motor oil.
Base your reasoning not on memorization but rather upon what you know about molecular interactions and the various physical properties of gasoline and motor oil.
Definitions:
Related Questions
Q19: If you had a 1 M solution
Q30: What is the difference between a dipole-dipole
Q74: Which type of radiation is being emitted
Q79: What is the relationship between the light
Q82: When is the geometry of a molecule
Q86: Which of the following statements best describes
Q110: The main component of bleach is sodium
Q114: Two candles of the same mass, one
Q126: Where does the earth receive most of
Q131: Why might a solvent like turpentine be