Based on atomic size, which would you expect to be more soluble in water: helium, He, or nitrogen, 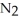 ?
?
Definitions:
Cyclopentanone
A five-membered cyclic ketone, characterized by a ring structure containing one ketone group, used as a precursor in the synthesis of fragrances and pharmaceuticals.
Hydrate
A compound typically formed by the absorption of water molecules into its structure, often resulting in a crystalline form.
PhCOCl
PhCOCl refers to benzoyl chloride, a colorless, fuming liquid with a pungent smell, used in organic synthesis, represented by the formula C6H5COCl.
Carbonyl Group
A functional group consisting of a carbon atom double-bonded to an oxygen atom (C=O) found in aldehydes, ketones, carboxylic acids, and esters.
Q53: What is an acid?<br>A) anything that donates
Q54: What would the concentration of OH<sup>-</sup> be
Q120: What is the mass of a water
Q123: Why does condensation have a tendency to
Q124: Would it be easier or harder for
Q125: After a uranium fuel rod reaches the
Q129: Hydrogen chloride is added to a buffer
Q135: The energy level diagram ranks orbitals by
Q139: If the solubility of a compound is
Q141: Which of the following molecules should have