Account for the observation that ethyl alcohol, 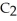
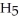 OH, dissolves readily in water but dimethyl ether, C
OH, dissolves readily in water but dimethyl ether, C 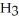 OC
OC 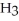 , which has the same number and kinds of atoms, does not.
, which has the same number and kinds of atoms, does not. 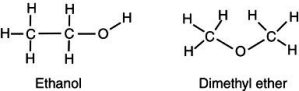
Definitions:
Altered States
Conditions of consciousness that are significantly different from normal waking consciousness, often induced by drugs, meditation, hypnosis, or extreme stress.
Waking Consciousness
State in which thoughts, feelings, and sensations are clear and organized and the person feels alert.
Hyperconsciousness
An increased awareness or preoccupation with one's own thoughts, feelings, or sensory experiences.
Sleep Disorder
A medical disorder that impacts one's sleep patterns, potentially causing significant distress and difficulties in daytime functioning.
Q7: Which of the following molecules contains a
Q16: A sample of steel is composed of
Q19: Which of the following atoms is the
Q28: Chlorine, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg" alt="Chlorine, ,
Q47: Which of the following describes the reason
Q60: Oxygen, O, (number 8), sulfur, S, (number
Q62: Why does transforming water from 100°C liquid
Q74: What is the hydroxide ion concentration in
Q94: Which of the following solutions is the
Q152: Friends on a crowded ice skating rink