Why does water not freeze at 0°C when either ions or molecules other than 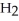 O are present?
O are present?
Definitions:
Federal Taxation
The system by which the federal government of a country levies taxes on individuals and corporations to fund its operations.
Inelastic
Describes a situation where the demand or supply of a good or service is relatively unresponsive to changes in price.
Excise Tax
A tax levied on specific goods, services, or transactions, often included in the price of the product, such as alcohol, tobacco, and gasoline.
Borne
The bearing or enduring of costs, responsibilities, or consequences by an entity, often in the context of who ultimately pays for or suffers from economic decisions.
Q24: Which of the following compounds has polar
Q34: When the isotope bismuth-213 emits an alpha
Q51: If the following generic atom were to
Q59: Which of the following professions would likely
Q60: Which of the above illustrations shows a
Q65: Complete the following nuclear equation: ?? →
Q82: When is the geometry of a molecule
Q111: If carbonic acid (H<sub>2</sub>CO<sub>3</sub>) were to undergo
Q121: Glucose, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg" alt="Glucose, ,
Q128: According to the following reaction, which molecule