Why is calcium chloride, 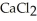 , more effective at melting ice than sodium chloride, NaCl?
, more effective at melting ice than sodium chloride, NaCl?
Definitions:
Shallow Deposits
Geological accumulations of minerals, sediments or other materials that are located near the surface of the Earth.
Separate the Metal
The process of extracting a metal from its ore or mixture, often involving physical or chemical separation techniques.
Outermost Zone
A term typically referring to the furthest or most remote area within a particular boundary or geographical region.
Metamorphic Rock
Rock formed from the transformation of existing rock types through high pressure, high temperature, or both, without melting into liquid magma.
Q7: A buffer solution can neutralize _.<br>A) strong
Q41: The following set of redox reactions takes
Q69: Which of the following molecules is most
Q92: Mercury forms a convex meniscus with glass
Q114: What is the temperature change if you
Q114: If Uranium-234 decays via alpha emission, what
Q124: Which covalent bond is more polar: a
Q126: Ice floats in room temperature water, but
Q128: How many substituents are on the following
Q140: Why is heat often added to chemical