Why does water not freeze at 0°C when either ions or molecules other than 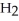 O are present?
O are present?
Definitions:
Binding Price Floor
A government-imposed price control or limit on how low a price can be charged that is above the market equilibrium price, leading to a surplus.
Market For Milk
A specific commodity market involving the buying and selling of milk, influenced by supply and demand dynamics, production costs, and regulatory factors.
Variable Ratio Schedules
A reinforcement schedule in operant conditioning where a response is reinforced after an unpredictable number of responses, leading to high and steady response rates.
Reinforcement Theory
A behavioral theory proposing that behavior is a function of its consequences, with behaviors being encouraged or discouraged through positive or negative reinforcement.
Q11: What is the main difference between a
Q19: For the above energy profiles, which reaction
Q20: How does n-type silicon differ from pure
Q45: If you mix a typical iodine ion
Q62: Which of the following intermolecular forces best
Q75: For the following reaction, identify whether the
Q87: Water, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg" alt="Water, O,
Q118: Wild plants readily grow "all by themselves"
Q132: What is the formula mass of a
Q134: What needs to be done to convert