How many grams of water, 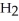 O, and propene,
O, and propene, 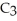
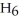 , can be formed from the reaction of 6.0 g of 2-propanol,
, can be formed from the reaction of 6.0 g of 2-propanol, 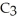
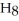 O?
O? 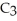
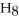 O →
O → 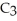
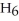 +
+ 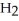 O 2-Propanol Propene Water
O 2-Propanol Propene Water
Definitions:
Real Estate
Property consisting of land and the buildings on it, along with its natural resources.
Legal Position
The stance or perspective held on legal matters, often determined by interpreting laws, precedences, and facts.
Conduct of Parties
The actions, behavior, and decisions of those involved in a legal agreement or dispute, which might influence the outcome.
Agent's Authority
Refers to the scope of power granted to an agent by the principal, allowing the agent to act on behalf of the principal in specific matters.
Q24: Heat is absorbed by a substance when
Q27: An adhesive force is best described as
Q41: Which of the above liquids would most
Q57: Which of the above liquids has the
Q77: Germanium chloride, Ge <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg" alt="Germanium chloride,
Q88: How many substituents are on the following
Q101: If the relative mass of a hydrogen
Q118: Which of the following statements does not
Q129: Why does water have a high specific
Q146: In a chemical reaction, the bonds being