How many moles of water, 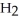 O, are produced from the reaction of 16 grams methane, C
O, are produced from the reaction of 16 grams methane, C 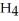 , with an unlimited supply of oxygen,
, with an unlimited supply of oxygen, 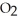 . How many grams of
. How many grams of 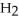 O is this? C
O is this? C 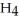 + 2
+ 2 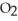 → C
→ C 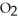 + 2
+ 2 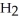 O
O
Definitions:
Stereochemical Detail
Refers to the three-dimensional aspects of molecule structures, including how atom connectivity impacts chemical reactivity and physical properties.
Chirality Center
An atom, typically carbon, in a molecule that has four different substituents and can exist in two enantiomeric forms, leading to different spatial configurations.
Stereocenter
An atom in a molecule that is bonded to four different groups, creating different spatial arrangements.
Enantiomer
Molecules that are mirror images of each other and cannot be superimposed onto each other, commonly found in chiral molecules.
Q5: Which of the following best describes a
Q16: Which of the following molecules contains a
Q21: Why does a lake freeze from the
Q39: Given the following generic chemical reaction, which
Q45: A major source of chlorine gas, <img
Q65: Why do ice skates work?<br>A) The pressure
Q67: What was the first polymeric hydrocarbon extensively
Q80: You are given two samples of elements,
Q119: From the schematic above: why do nonpolar
Q150: What is the main difference between High