How many moles of water, 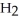 O, are produced from the reaction of 16 grams methane, C
O, are produced from the reaction of 16 grams methane, C 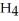 , with an unlimited supply of oxygen,
, with an unlimited supply of oxygen, 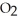 . How many grams of
. How many grams of 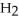 O is this? C
O is this? C 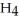 + 2
+ 2 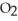 → C
→ C 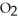 + 2
+ 2 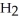 O
O
Definitions:
Childhood Origins
Refers to the initial stages or causes of behaviors, mental processes, or conditions that begin or develop during childhood.
Free Association
A psychoanalytic technique in which a patient says whatever comes to mind without censorship, used to explore the unconscious.
Unconscious Processes
Psychological activities and content not within the immediate awareness of the individual, influencing behavior and perceptions.
Psychotherapist
A trained professional who treats mental health problems and emotional difficulties through the use of psychological techniques and verbal communication.
Q17: Which of the following compounds is likely
Q25: Assume air has an average molar mass
Q49: The number of nonbonding pairs in the
Q50: Which of the following substances contains <img
Q65: What is the purpose of the salt
Q90: How are oxygen molecules attracted to water
Q105: In a battery, the following two oxidation-reduction
Q110: How is the solubility of a solid
Q113: Hydrogen chloride, HCl, is a gas at
Q120: Which of the above stick structures would