Multiple Choice
Use the bond energies below to determine whether the following reaction is exothermic or endothermic: 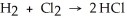 H-H (bond energy: 436 kJ/mol) Cl-Cl (bond energy: 243 kJ/mol)
H-H (bond energy: 436 kJ/mol) Cl-Cl (bond energy: 243 kJ/mol)
H-Cl (bond energy: 431 kJ/mol)
Definitions:
Related Questions
Q4: Which of the following is the simplest
Q8: Why do ice cubes get smaller when
Q26: How many moles of sugar, C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>, are
Q47: How does the combined volume of the
Q56: Which of the following would have the
Q64: Which of the following statements describes an
Q72: Which of the following statements describes a
Q73: What would be the shape of a
Q125: Given the following diagram, describe what happens
Q146: Which of the following would have the