Why does iodine, 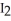 (s) , spontaneously sublime at room temperature?
(s) , spontaneously sublime at room temperature?
Definitions:
Imaginary Friends
Non-real companions created by children, often serving as a way to explore social relationships and emotions.
Cognitive Problems
Difficulties with processes related to thinking, including memory, perception, problem-solving, and decision-making.
Real Relationships
Authentic connections between individuals, characterized by honesty, trust, and mutual respect.
Animism
The attribution of life and intentionality to inanimate objects.
Q15: What would the formula be for the
Q42: Which of the following species is the
Q52: How might you tell whether or not
Q61: How many electrons are transferred from iron
Q67: A solid has a solubility at room
Q80: Which of the following compounds is an
Q118: Why is oxygen usually an oxidizing agent?<br>A)
Q119: When a hydronium ion concentration equals 1
Q138: What was the first polymer that was
Q147: How many electrons are used to draw