How readily an acid donates a hydrogen ion is a function of how well the acid is able to accommodate the resulting negative charge it gains after donating. Which should be the stronger acid: water or hypochlorous acid? Why? 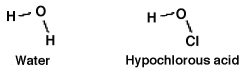
Definitions:
Words and Phrases
Specific terms or expressions used within a particular context or field, often with defined meanings.
Case Digest
A summarized compilation of legal case decisions that includes the important facts, issues, and holdings, intended to aid in legal research.
Research Tool
A resource or device used to gather information, analyze data, or facilitate investigation into a particular subject or area of inquiry.
Considered Law
Refers to principles that are acknowledged and taken into account in legal reasoning and judgments.
Q3: The following statement describes what level of
Q16: A sample of steel is composed of
Q25: Which of the following compounds could never
Q42: Why can you determine wind direction by
Q42: Which of the structures shown below are
Q43: Qualitatively, what happens to the hydronium ion
Q50: Surface tension is the _.<br>A) tendency for
Q70: It is important to protect water pipes
Q84: Which of the following elements would be
Q146: In a chemical reaction, the bonds being