Does a water molecule become more or less polar when bound to a metal ion, such as 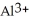 ? Why? (Hint: How might the electrons of the O-H covalent bonds in water "feel" about the highly charged aluminum ion?)
? Why? (Hint: How might the electrons of the O-H covalent bonds in water "feel" about the highly charged aluminum ion?)
Definitions:
Acronym
An abbreviation formed from the initial letters of other words and pronounced as a word.
Horizontal Axis
A straight line that runs left and right across a graph or chart, typically used as a reference for measuring and orienting features.
Reference Axis
An axis in geometric dimensioning and tolerancing (GD&T) that serves as a baseline for the measurement of other features.
Point-To-Point Programming
A method used in computer numerical control (CNC) machining where commands guide a tool from one point to another, minimizing non-productive time.
Q17: Which of the following compounds is likely
Q34: What is the primary difference between a
Q55: The warming of a saturated solution of
Q65: What is the environmentally unfriendly component of
Q109: How many structural isomers are there for
Q123: Which of the above illustrations shows a
Q128: What is the mass of one mole
Q131: Given the following generic chemical reaction, which
Q131: Which region (a-b-c) is an alcohol functional
Q136: A single carbon branch within a hydrocarbon