Multiple Choice
Why are aqueous solutions of highly charged metal ions, such as 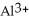 , usually acidic?
, usually acidic?
Definitions:
Related Questions
Q3: The following statement describes what level of
Q5: The oxidation of iron to rust is
Q28: What might the relationship be between an
Q51: Why is the H-F bond so much
Q83: Which element is closer to the upper
Q87: Why is it that in cold winters
Q108: An acid and a base react to
Q126: A battery operates by _.<br>A) oxidation<br>B) reduction<br>C)
Q133: Balance the following equation. _ NO →
Q147: What is the sum of the atomic