Which should be a stronger base: ammonia, 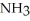 , or trifluoronitrogen,
, or trifluoronitrogen, 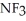 ? Why?
? Why? 
Definitions:
P(X = 1)
The probability that a random variable X takes the value 1, a specific outcome in a probability distribution.
Sports Fans
Individuals who exhibit enthusiasm and support for sports teams or athletes, often through attendance at games, viewership, and merchandise purchase.
College Students
Individuals enrolled in an institution of higher education with the aim of achieving a degree, certificate, or course completion.
Binomial Distribution
A probability distribution that summarizes the likelihood that a value will take one of two independent states under a given number of parameters.
Q5: From the diagram above describing the phase
Q25: A soap molecule is _.<br>A) primarily polar<br>B)
Q27: Hydrocarbons with a carbon-carbon double bond are
Q51: Why is the H-F bond so much
Q67: Sodium metal is _.<br>A) oxidized in the
Q84: Tertiary protein structure is determined by _.<br>A)
Q92: Which of the following picture might best
Q102: What is a functional group?<br>A) a set
Q133: What happens when the molecule-to-molecule attractions in
Q146: Which of the following would have the