When a hydronium ion concentration equals 1 × 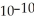 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
moles per liter, what is the pH of the solution? Is the solution acidic or basic?
Definitions:
Return Policy
A set of rules established by a company outlining how customers can return or exchange unwanted or defective merchandise they've purchased.
Retailer's
Pertaining to businesses that sell goods directly to consumers for personal use, operating from physical or online locations.
Cognitive Dissonance
A psychological phenomenon where an individual experiences distress due to holding conflicting beliefs, attitudes, or values.
Psychological Tension
Internal conflict or stress experienced by individuals due to opposing thoughts, beliefs, or feelings.
Q4: For the following reaction, identify whether the
Q11: Iron is useful for reducing copper ions
Q20: Which of the following is a correctly
Q33: Which of the following statements describes a
Q43: Which of the above reactions would proceed
Q69: What is the main compound formed in
Q75: Starch is a polysaccharide that _.<br>A) is
Q84: Which of the following is an aromatic
Q85: Would you expect to find more dissolved
Q126: Ice floats in room temperature water, but