When a hydronium ion concentration equals 1 × 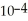 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
moles per liter, what is the pH of the solution? Is the solution acidic or basic?
Definitions:
Net Exports
The value of a country's total exports minus its total imports, representing the net trade balance of goods and services.
Interest Rates
The share of a loan levied as interest on the borrower, typically indicated as an annual percentage of the unpaid loan amount.
Twin Deficits
Refers to the situation where a country is running both a fiscal deficit (government spending exceeds revenue) and a current account deficit (imports exceed exports).
Budget Deficit
The condition when a government spends more than its income, requiring it to borrow money or accumulate debt to cover the gap.
Q31: Might reverse osmosis be used to obtain
Q35: The following diagram depicts the reaction between
Q36: A refrigerator delays the spoilage of food
Q45: How many grams of sodium chloride are
Q63: A saturated solution of compound X in
Q89: What was the most important discovery that
Q89: Many solvents expand to occupy greater volumes
Q101: Describe what usually happens to a hot
Q104: Which of the following statements about strong
Q131: Given the following generic chemical reaction, which