How readily an acid donates a hydrogen ion is a function of how well the acid is able to accommodate the resulting negative charge it gains after donating. Which should be the stronger acid: water or hypochlorous acid? Why? 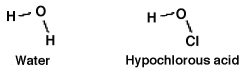
Definitions:
Nursing Research
The scientific process that validates and refines existing knowledge and generates new knowledge that directly and indirectly influences nursing practice.
Negative Attitude
A pessimistic viewpoint or mindset that tends to see the worst aspects of situations or expect the most unfavorable outcomes.
Lack of Support
Refers to the absence of assistance, encouragement, or resources from others when needed.
TENS
Transcutaneous Electrical Nerve Stimulation, a therapy using low-voltage electrical currents to relieve pain by stimulating nerves.
Q1: Southern Asia is marked by a wildly
Q4: How does a catalyst increase the rate
Q38: Upon ingestion, grain alcohol, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg" alt="Upon
Q40: According to the following reaction, which molecule
Q64: Why are the melting temperatures of most
Q78: If water had a lower specific heat,
Q83: What do all fats have in common?<br>A)
Q84: Which of the following elements would be
Q129: Hydrogen chloride is added to a buffer
Q138: What was the first polymer that was