The amphoteric reaction between two water molecules is endothermic, which means that this reaction requires the input of energy in order to proceed: Energy + 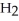 O +
O + 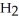 O →
O → 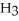
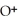 + O
+ O 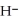 The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of
The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of 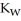 be expected to increase, decrease, or stay the same with increasing temperature?
be expected to increase, decrease, or stay the same with increasing temperature?
Definitions:
Evolution
The gradual development or change of something over a period of time, which can refer to biological organisms, concepts, or systems.
IDEATE Method
A step in the design thinking process that focuses on generating a wide range of ideas and solutions to address a defined problem.
Food Truck
A mobile kitchen set up in a vehicle, serving food to the public at various locations.
Opportunity Recognition
The ability to identify and evaluate potential business or investment opportunities within the environment.
Q2: Lakes lying in granite basins, such as
Q4: Why is a large quantity of water
Q11: Which region (a-b-c) is a hydroxyl functional
Q24: Formaldehyde is a toxic preservative with the
Q27: The general outcome of catabolism is _.<br>A)
Q42: Why can you determine wind direction by
Q58: Which of the following elements would be
Q86: According to the figure above, which amino
Q112: What happens to the pH of a
Q127: Which of the above stick structures would