When the pH of a solution is 1, the concentration of hydronium ions is 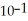 M, which is the same as 0.1 M. Assume that the volume of this solution is 500 mL and that the solution is not buffered. What would be the new pH of this solution after 500 mL of pure water is added? You will need a calculator with a log function to answer this question.
M, which is the same as 0.1 M. Assume that the volume of this solution is 500 mL and that the solution is not buffered. What would be the new pH of this solution after 500 mL of pure water is added? You will need a calculator with a log function to answer this question.
Definitions:
Peripheral Integration
The process by which peripheral or less developed areas are incorporated into the global economy, influencing local development and connectivity.
Ecolonization
Refers to the process where digital technology and internet platforms are used to exert economic and cultural influences across borders.
Authoritarian Governments
Political systems characterized by a concentration of power in a leader or small elite not constitutionally responsible to the public.
Basic Needs
Fundamental requirements necessary for individuals to maintain a healthy and sustainable life, typically including food, water, shelter, and clothing.
Q3: The following statement describes what level of
Q16: What is one of the advantages of
Q20: Shown below is the line structure of
Q25: Assume air has an average molar mass
Q28: The most complicated protein structure is the
Q34: For the following reaction, identify whether the
Q56: Some people fear drinking distilled water because
Q73: What is an obvious physical difference between
Q151: Which of the following polymers has the
Q154: Which of the following polymers would you