What element is oxidized in the following equation and what element is reduced? 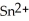 + 2 Ag → Sn + 2 Ag⁺
+ 2 Ag → Sn + 2 Ag⁺
Definitions:
Task Group
A group of individuals gathered to achieve a specific goal or task, often within an organizational setting.
Agenda Items
Specific topics, issues, or tasks listed for discussion or action in a meeting's agenda.
Leader
An individual who guides or directs a group, organization, or country, often demonstrating qualities such as vision, courage, and integrity.
Win-lose Strategy
A conflict resolution approach where one party's gain is perceived as another party's loss, leading to a competitive scenario.
Q27: An adhesive force is best described as
Q31: Might reverse osmosis be used to obtain
Q49: Why do water drops bead on a
Q61: Fourteen grams of nitrogen, N<sub>2</sub>, (N, atomic
Q68: Should the specific heat of your automobile's
Q77: Which of the following statements describes a
Q82: Sodium hydroxide is added to a buffer
Q86: Which of the following statements best describes
Q94: The coiling of a chain of amino
Q132: What is the formula mass of a