What element is behaves as the oxidizing agent in the following equation and what element behaves as the reducing agent? 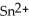 + 2 Ag → Sn + 2 Ag⁺
+ 2 Ag → Sn + 2 Ag⁺
Definitions:
Communication Barriers
Obstacles in the process of communication that prevent effective exchange of ideas or information.
Communication Effectiveness
The degree to which communication achieves its intended outcome efficiently and effectively.
Intended Message
The message that an individual or organization aims to convey through their communication.
Written Communications
Refers to the process of conveying messages or information through written forms, such as emails, reports, and memos.
Q10: What determines the chemical and physical properties
Q13: If champagne has a proof of 28,
Q50: Surface tension is the _.<br>A) tendency for
Q68: Which of the following solutions is the
Q85: Why are aqueous solutions of highly charged
Q93: Why is phosphoric acid, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg" alt="Why
Q99: Three identical candles are placed in the
Q104: Why does evaporation cool a liquid?<br>A) The
Q129: Small samples of oxygen gas needed in
Q131: Why might a solvent like turpentine be