Hydrogen sulfide, H2S, burns in the presence of oxygen, 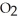 , to produce water,
, to produce water, 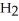 O, and sulfur dioxide, SO2. Through this reaction, is sulfur oxidized or reduced? 2
O, and sulfur dioxide, SO2. Through this reaction, is sulfur oxidized or reduced? 2 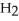 S + 3
S + 3 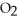 → 2
→ 2 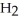 O + 2
O + 2 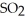
Definitions:
Industrialization
The process of transforming economies from primarily agricultural to one based on the manufacturing of goods, characterized by technological innovation and increased productivity.
Industrialized West
Refers to Western countries that have undergone industrialization, characterized by economic development, advanced technology, and a shift from agricultural to manufacturing and service industries.
Agricultural Development
The strategic enhancement of agricultural productivity and the expansion of farming practices to meet increasing food demands.
Modernization
The process of adopting modern ways of life and ideas, often involving technological advancements, urbanization, and social and economic changes.
Q8: One of the active components of marijuana,
Q17: How do fats compare to other biomolecules
Q22: What role do CFCs play in the
Q59: Which of the following is not one
Q67: What was the first polymeric hydrocarbon extensively
Q82: At one time, halothane, C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg"
Q103: Which of the following statements best describes
Q114: What is the main function of the
Q118: Why might an area with a large
Q138: Why is the anode of a battery