Consider the following molecules. What is the relationship between the degree to which the molecule is oxidized and its polarity? 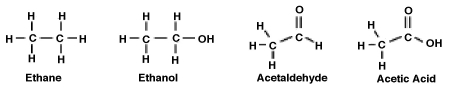
Definitions:
Ampacity
The maximum amount of electric current a conductor or device can carry before sustaining immediate or progressive deterioration.
Circular Mils
A unit of area used specifically in the calculation of wire cross-sectional areas, defined as the area of a circle with a diameter of one mil (one-thousandth of an inch).
Diameter
A straight line passing from side to side through the center of a body or figure, especially a circle or sphere.
MEGGER
A brand name that has become a generic term for insulation resistance testers, which measure the resistance of electrical insulation.
Q6: The following set of redox reactions takes
Q7: Which of the following statements accurately describes
Q8: Why do ice cubes get smaller when
Q9: If you were throwing a party, which
Q12: Which of the above liquids would most
Q33: What is the difference between a beta
Q40: A change in configuration, but not a
Q42: Why is it important for a chemist
Q76: A drug that alters our tolerance to
Q88: Suppose that water is used in a