Consider the following molecules. What is the relationship between the degree to which the molecule is oxidized and its polarity? 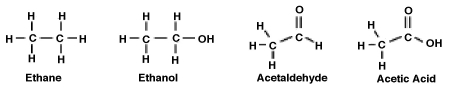
Definitions:
Lens
A transparent structure in the eye that focuses light rays onto the retina to form clear images.
Retina
A light-sensitive layer at the back of the eye that converts light into electrical signals sent to the brain.
Keratoconjunctivitis
An inflammation of the cornea and conjunctiva of the eye, often resulting from infection, dry eyes, or exposure to irritants.
Kerat/o
A prefix in medical terms related to cornea or keratin.
Q2: Why do heavy drinkers have a greater
Q4: Suggest why withdrawal symptoms are observed after
Q35: Based on their relative positions in the
Q38: Why might a change in pH cause
Q79: What are the formula masses of water,
Q109: Why is the anode of a battery
Q112: What products are formed upon the reaction
Q125: Condensation is _.<br>A) always a cooling process<br>B)
Q134: Which of the following statements regarding a
Q142: Which of the above molecules would most